A new study published in npj Women’s Health reveals how heart rate and heart rate variability follow distinct circadian rhythms throughout pregnancy, captured continuously in real life using wearable devices.
By following pregnant women from the second trimester to delivery, the researchers uncovered patterns that were previously invisible.
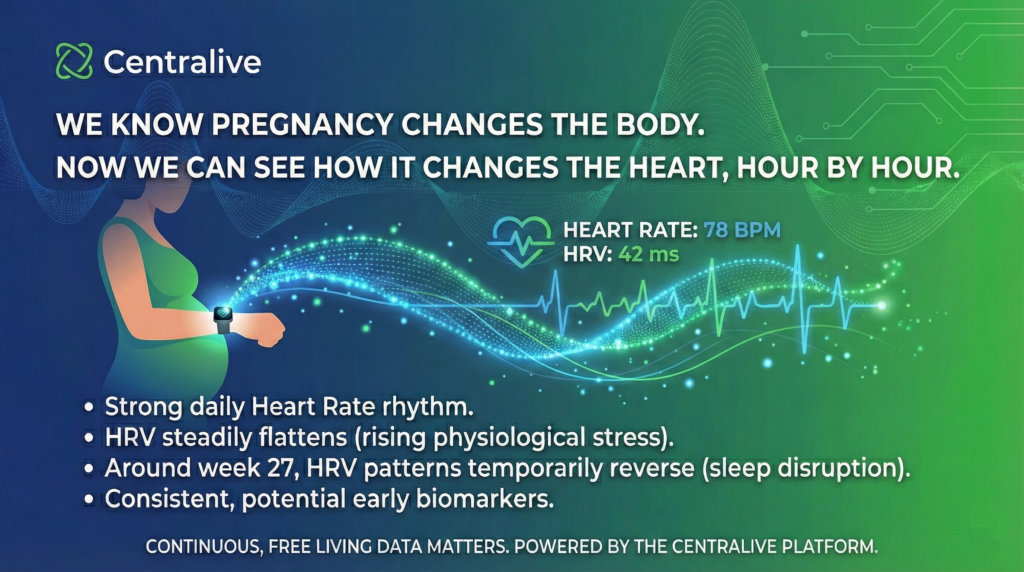
𝗪𝗵𝗮𝘁 𝘀𝘁𝗼𝗼𝗱 𝗼𝘂𝘁:
• Strong daily heart rate rhythms persist across pregnancy
• Heart rate variability steadily flattens, reflecting rising physiological stress
• Around week 27, HRV patterns temporarily reverse, pointing to elevated nighttime stress and sleep disruption
• These rhythms are consistent across individuals, highlighting potential early biomarkers
Study design and analytical approach
The study followed 30 low-risk pregnant women from early second trimester through delivery, collecting continuous photoplethysmography-derived HR and HRV data via smartwatches. Rather than relying on resting or clinic-based measures, data were captured during daily activities and sleep, enabling a realistic assessment of autonomic dynamics.
A key methodological contribution lies in the signal processing pipeline. Machine learning models were used both to extract reliable HR and HRV metrics from noisy wearable data and to impute missing samples, which are unavoidable in long-term free-living studies. Circadian rhythms were then quantified using population-mean Cosinor analysis, yielding interpretable rhythm parameters: MESOR (rhythm-adjusted mean), amplitude, and acrophase.
Divergent circadian trajectories of HR and HRV
HR exhibited statistically significant 24-hour rhythmicity across nearly all gestational weeks studied. The HR MESOR increased steadily from week 14 through approximately week 34, consistent with rising cardiovascular demand, before declining toward term. HR amplitude decreased across the second trimester and rebounded in the third, while acrophase remained relatively stable, suggesting preserved circadian phase despite changing physiological load.
In contrast, HRV, assessed primarily via RMSSD, showed progressive attenuation of both MESOR and amplitude across pregnancy. Circadian rhythmicity in HRV weakened notably in late gestation, indicating flattening of day–night variation and reduced autonomic flexibility. These findings align with sympathetic predominance and increasing physiological stress as pregnancy advances.
A notable observation emerged around week 27, where group-level HRV rhythms exhibited a phase inversion, with lower HRV during nighttime hours. Although heterogeneous at the individual level, this pattern suggests elevated nocturnal autonomic stress, potentially linked to worsening sleep fragmentation and discomfort as pregnancy transitions into the third trimester.
Inter-individual variability and robustness
While MESOR and amplitude estimates were relatively stable across participants, acrophase showed substantial inter-individual variability for both HR and HRV, on the order of several hours. Sensitivity analyses using leave-one-out cross-validation demonstrated that population-level rhythm estimates were robust and not driven by individual outliers. Maternal age was modestly associated with delayed HRV acrophase but not with MESOR or amplitude.
Implications for pregnancy research and digital phenotyping
By establishing normative circadian profiles of HR and HRV across pregnancy, this work provides a critical reference framework for future studies of pregnancy complications. Circadian rhythm parameters may offer added sensitivity over traditional averages, particularly for detecting early dysregulation associated with conditions such as preeclampsia or preterm birth.
More broadly, the study illustrates the feasibility and value of combining wearable sensing, machine learning, and circadian modeling in longitudinal maternal health research. As digital phenotyping matures, circadian biomarkers derived from continuous monitoring may enable earlier risk stratification, improved mechanistic insight into autonomic adaptation, and time-aware interventions in prenatal care.
👏 Congratulations to the authors:
Mahkameh Rasouli, PhD(c), RN, CRNA, Mohammad Feli, Iman Azimi, Shahab Haghayegh, Fatemeh Sarhaddi, Hannakaisa Niela-Vilen, Anna Axelin, Pasi Liljeberg, Amir M. Rahmani
Data collection for this study was powered by the Centralive platform, enabling continuous monitoring and real-world physiological insight.




